DQ, IQ, OQ for Cleanroom & HVAC System
Qualification process ensuring design, installation, operation, and performance compliance with ISO 14644, WHO GMP, PIC/S.
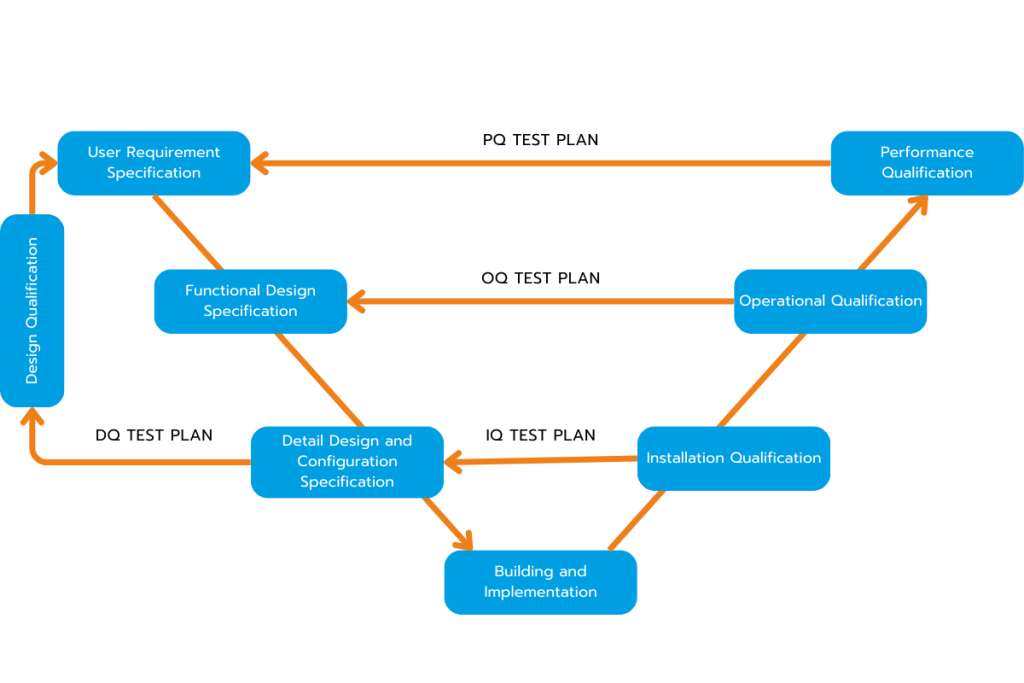
What is DQ, IQ, OQ, PQ ?
The Qualification Process verifies that cleanroom and HVAC systems are designed, installed, and operated as intended, meeting regulatory and performance requirements. It is composed of four stages: DQ, IQ, OQ, and PQ.
- Aligned with ISO 14644, WHO GMP, PIC/S, NEBB
- Covers Design → Installation → Operation → Performance
- Traceability: URS → Risk → Tests → Reports
Our Core Objectives
Design Verification Ensure design compliance with URS and standards.
Installation Accuracy Confirm correct installation and calibration.
Functional Testing Validate operational performance under controlled conditions.
Continuous Compliance Maintain validated state with periodic checks.
Where Our Services Make a Difference




Our Streamlined Process
DQ – Design Qualification: Verification that system design meets URS and ISO/GMP standards.
IQ – Installation Qualification: Ensure all components are installed correctly with traceable documentation.
OQ – Operational Qualification: Functional tests under defined conditions (airflow, pressure, particle count, T&H).
PQ – Performance Qualification: Confirm consistent performance during actual production operations.
Documentation & Standards
| Qualification Stage | Key Standard | Primary Deliverable |
|---|---|---|
| DQ | ISO 14644-1 / PIC/S | Design Qualification Report |
| IQ | WHO TRS 961 / ISO 14644-3 | Installation Qualification Report |
| OQ | ISO 14644-3 / NEBB | Operational Qualification Report |
| PQ | PIC/S PE009-16 / WHO TRS 961 | Performance Qualification Report |
Ready to Qualify Your Cleanroom?
Partner with QV TEST to achieve end-to-end qualification and regulatory compliance.
